Research
The MIGHTY Lab develops advanced methods at the intersection of signal processing, artificial intelligence, and optimization to address challenges in medical imaging. Our research emphasizes designing algorithms that enhance image quality, extract quantitative information, and enable reliable decision-making across diverse biomedical applications. We are particularly motivated by innovations that translate to practical, accessible technologies for improving healthcare delivery worldwide.
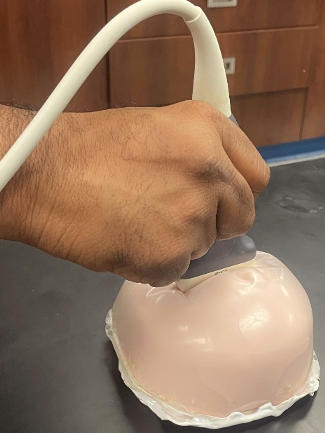
Strain Elastography
Ultrasound strain elastography measures tissue deformation under applied stress, offering unique insights into biomechanical properties that are not visible in conventional imaging. At the MIGHTY Lab, we integrate signal processing, optimization, and artificial intelligence to improve the accuracy, robustness, and usability of strain imaging. Our methods address challenges such as noise, motion artifacts, and variability in acquisition through advanced displacement tracking, adaptive filtering, and machine learning-based parameter selection.
We are particularly interested in creating quantitative strain maps that clinicians can trust across different tissues, imaging systems, and clinical environments. This includes developing algorithms that adapt to heterogeneous tissue properties, automate workflow decisions, and generate consistent biomarkers of tissue health. By advancing both theory and application, our aim is to translate elastography into a practical and accessible tool for disease detection, progression monitoring, and treatment evaluation worldwide.
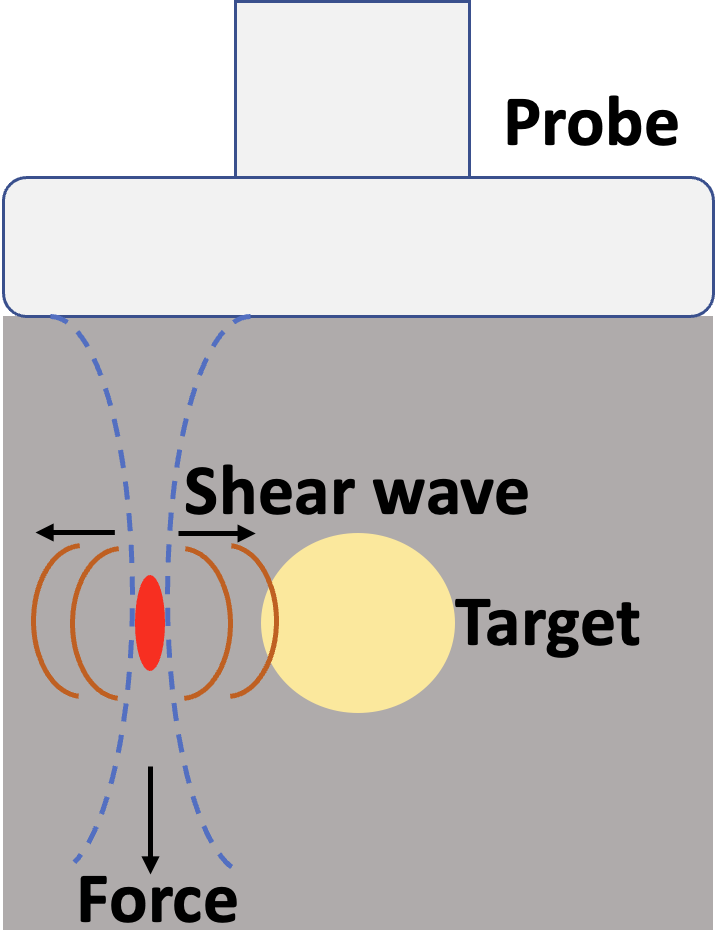
Shear Wave Elastography
Shear wave elastography measures the speed at which mechanically induced shear waves travel through tissue, providing a quantitative estimate of tissue stiffness. At the MIGHTY Lab, we develop advanced signal processing, optimization, and artificial intelligence methods to enhance the accuracy, robustness, and clinical utility of SWE. Our research focuses on improving wave speed reconstruction, reducing noise and motion sensitivity, and designing algorithms that adapt to complex tissue structures.
We aim to create reliable stiffness maps that can serve as objective biomarkers for disease detection, progression monitoring, and treatment assessment. By integrating physics-based modeling with machine learning, we are advancing SWE toward consistent, real-time, and widely accessible applications across a variety of clinical and global health settings.
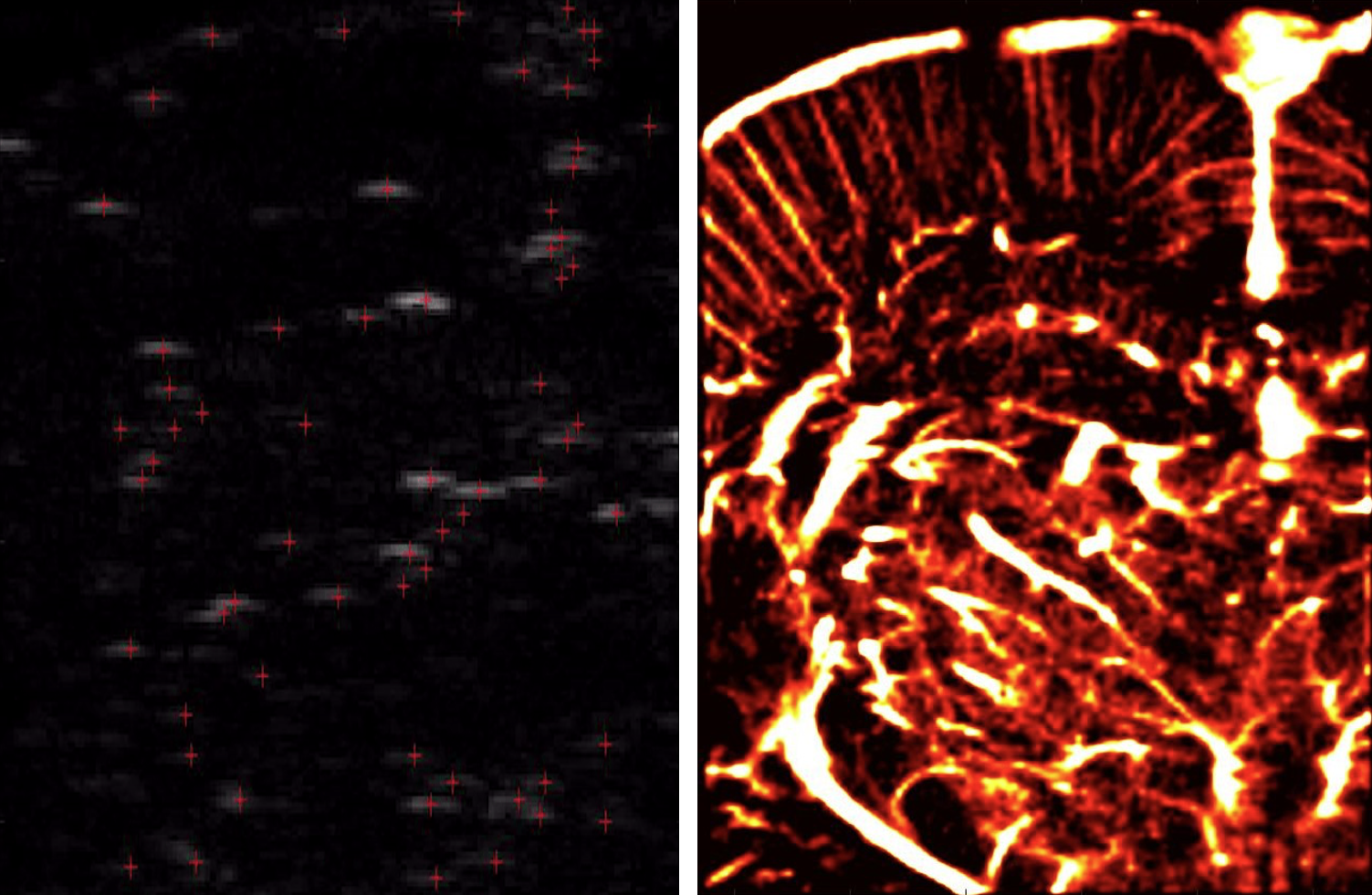
Super-Resolution Ultrasound
Super-resolution ultrasound imaging overcomes the diffraction limit of conventional ultrasound by localizing and tracking microbubbles at the capillary scale, enabling visualization of microvascular networks in unprecedented detail. At the MIGHTY Lab, we design advanced signal processing, optimization, and AI-based methods to enhance microbubble localization and tracking accuracy, improve temporal resolution, and reduce the impact of noise and motion. Our goal is to translate super-resolution ultrasound into a reliable tool for studying tissue microvasculature, quantifying disease-related changes, and supporting early diagnosis in both clinical and global health applications.
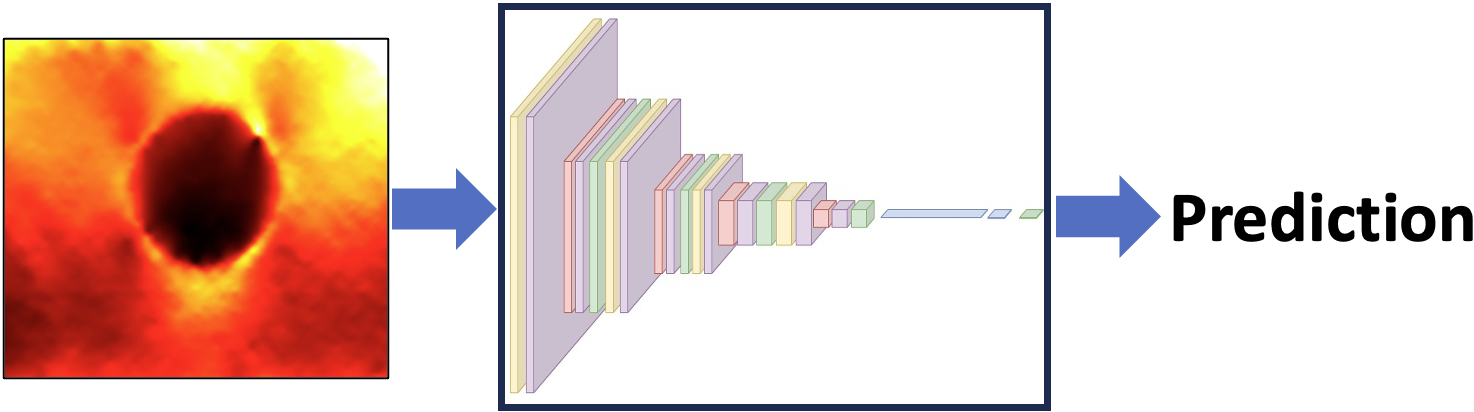
Medical Image Classification
Medical image classification enables automated recognition of patterns and features that support diagnosis, prognosis, and treatment planning. At the MIGHTY Lab, we develop AI-driven models that combine deep learning with domain-specific optimization to improve accuracy, interpretability, and robustness. Our work emphasizes building reliable classifiers that can provide trustworthy decision support in clinical practice.
Medical Image Segmentation
Medical image segmentation isolates anatomical structures or regions of interest, providing the foundation for quantitative analysis and clinical decision-making. At the MIGHTY Lab, we develop AI-based methods that improve boundary accuracy, robustness to noise, and consistency across patients. These approaches enable precise characterization of tissues and support downstream tasks such as biomarker extraction and disease monitoring.