Research

Virus-Host Dynamics and Biomolecular Condensates
Our research focuses on understanding how plant virus proteins, which undergo liquid-liquid phase separation (LLPS), contribute to virus accumulation and disrupt essential cellular processes. By investigating the role of these proteins in forming dynamic cellular structures, we aim to uncover how viruses manipulate host cells to enhance their spread. Additionally, we study cellular condensates that naturally form within plant cells, exploring their function in restricting viral accumulation and serving as a defense mechanism against viral infections.
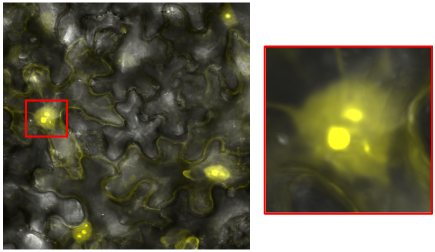
RNA virus interactions with the nucleus
RNA viruses primarily replicate in the cytoplasm, but research suggests that the nucleus may play a significant, yet poorly understood role in infection. We use live-cell imaging, transcriptomic analyses, and proteomic profiling to determine how viral proteins that enter the nucleus disrupt host nuclear pathways, gene regulation, transcription, and splicing. Understanding these interactions is critical for uncovering novel mechanisms of viral pathogenesis and host-virus dynamics.
Research Environment
The research environment at UMKC is excellent, with faculty regularly securing funding from major agencies like the National Institutes of Health (NIH), National Science Foundation (NSF), and the U.S. Department of Defense. In 2025, UMKC achieved the nation's top research designation, Carnegie Research 1 (R1). The Division of Biological and Biomedical Systems (BBS) within the School of Science and Engineering (SSE) promotes a strong culture of research success. Weekly seminars featuring ongoing departmental work, alongside presentations from prominent national and international scholars.
